Are you ready to experience innovation at its finest? Discover our cutting-edge SaaS platform designed for Regulatory, powered by the latest technology and hosted securely on the robust Azure platform. Whether you’re head of Regulatory, an IT business partner, or simply someone who appreciates top-tier performance, our web app is designed to exceed your expectations. Every solution we deliver is guided by tailored frameworks and proven methodologies, ensuring alignment with each client’s unique goals—without compromising industry standards or technical excellence
Are you ready to experience innovation at its finest?
DNXT Platform
Core Philosophy & Client Alignment
Enterprise-Grade Backend & Architecture
Modern Digital Experience (Frontend)
DNXT is dedicated to designing modern, high-performance digital experiences that are:
Fast, secure, and engaging across all platforms.
Built with architecture choices that prioritize scalability, reliability, and adaptability for seamless performance in any environment.
Intelligent Capabilities
AI-driven automation
Predictive analytics
Natural language processing (NLP)
These integrations drive smarter decision-making, personalized experiences, and improved operational efficiency.
Scalability:
Seamless Integration

01
DnXT RIM Suite
We believe Regulatory function to be a strategic capability of drug development as opposed to an administrative overhead. Our ambition is to shorten drug approval time from last clinical trial to first submission in under 2 weeks.
Our goal is to allow our customers to own a Regulatory SaaS system that seamlessly integrates with the marketplace (CROs, Affiliates). Flexibility to outsource parts of the regulatory operation by having total control of the end to end process. We have developed a platform that is intuitive, fast and just works.
02
DnXT Publisher
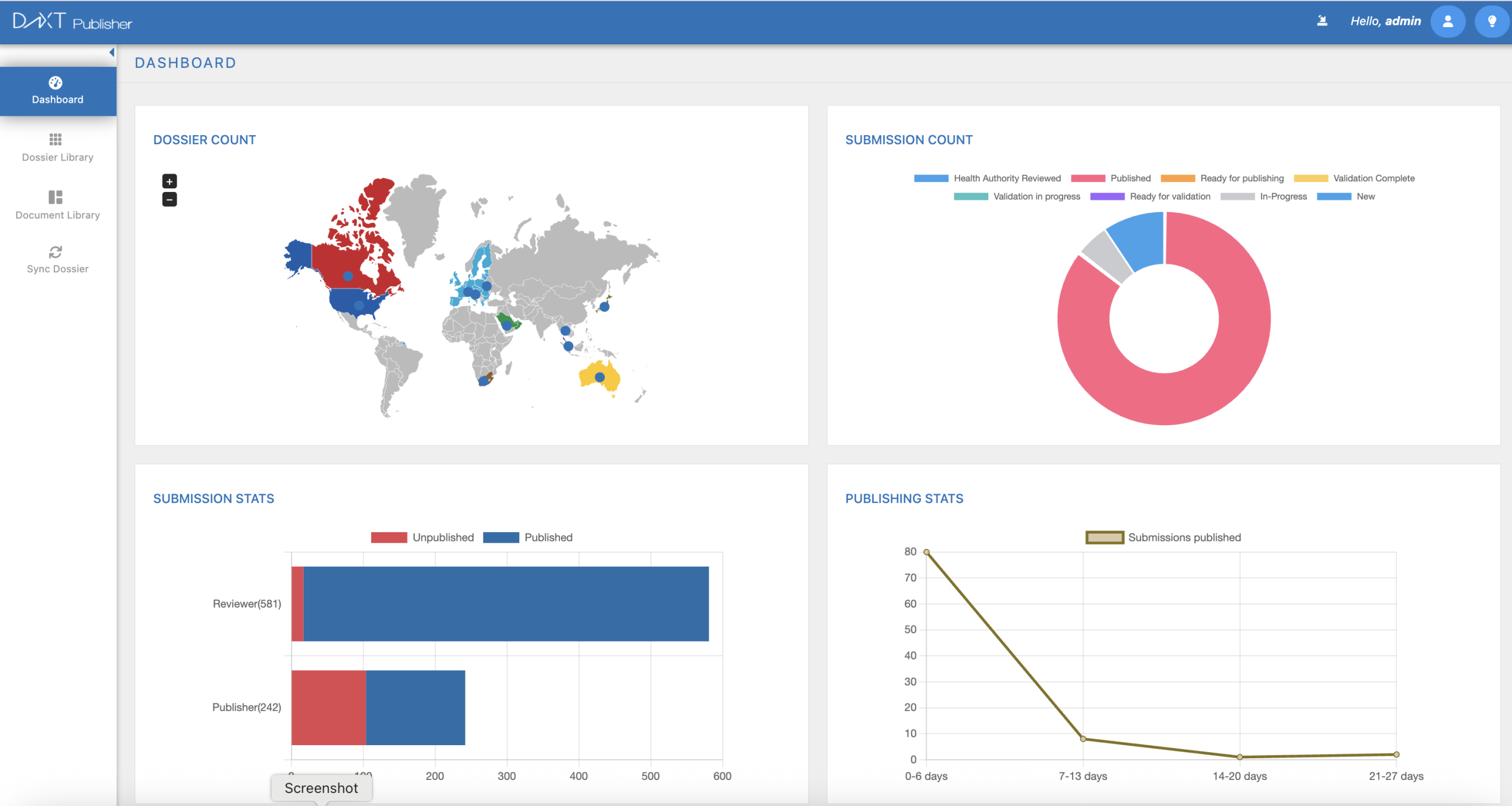
DnXT Publisher – Smarter, Faster eCTD Publishing
Take control of your regulatory submissions with DnXT Publisher, the intelligent eCTD publishing solution from DnXT Solutions. Purpose-built for life sciences, DnXT Publisher transforms complex dossier assembly into a streamlined, automated process — delivering compliant, submission-ready packages in record time.
With seamless integration to your DMS, real-time validation, and full support for global regulatory standards, your team can focus less on formatting and more on strategy. Whether you're submitting to the FDA, EMA, or beyond, DnXT Publisher helps you move faster, stay compliant, and scale with confidence.
Speed. Compliance. Simplicity. That’s the DnXT advantage.
03
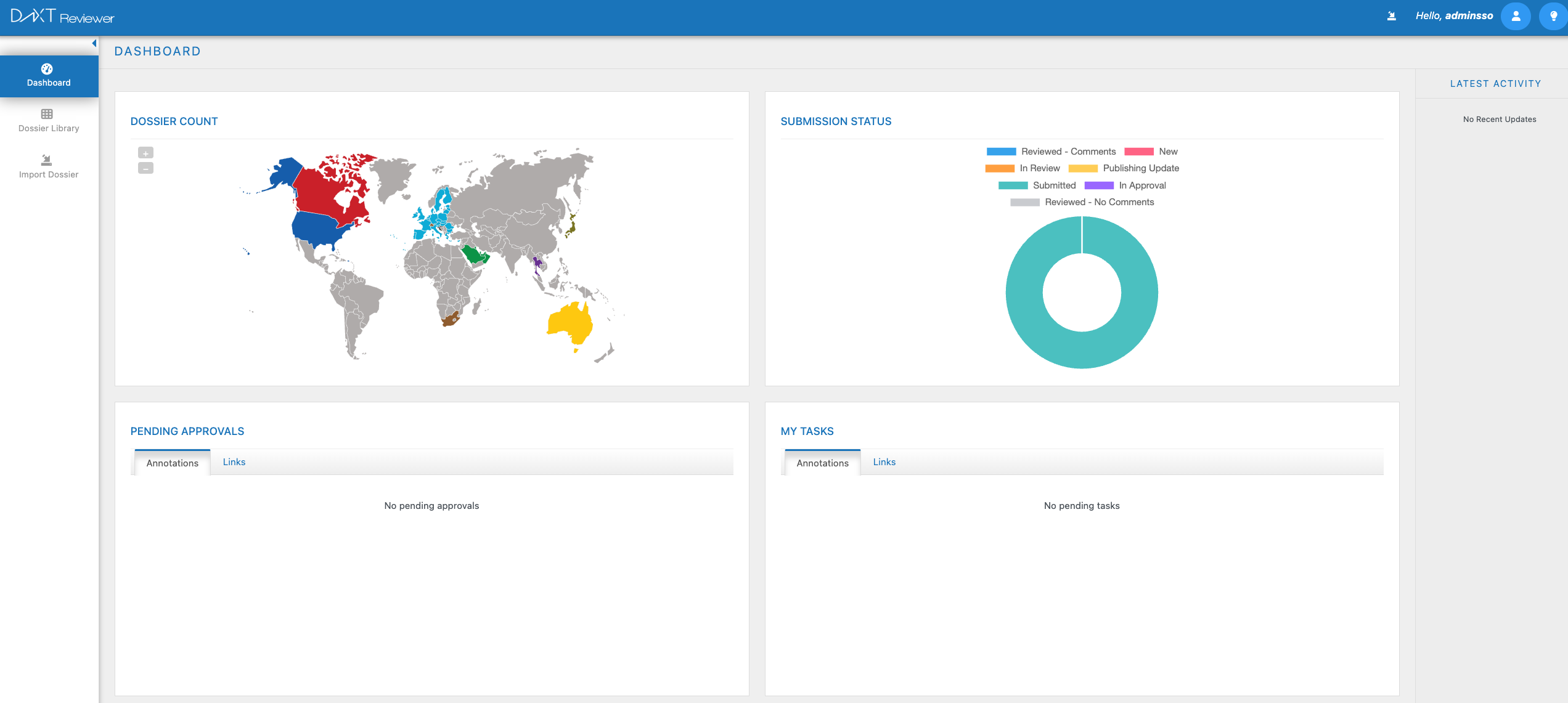
DnXT Reviewer
DnXT Reviewer — Redefining eCTD Review Excellence
Experience faster, smarter, and more compliant regulatory reviews with DnXT Reviewer, the next-generation eCTD reviewing solution designed for global life sciences teams.
Built on innovation and precision, DnXT Reviewer transforms complex eCTD submissions into a seamless, intuitive review experience. Navigate submissions effortlessly, compare sequences in seconds, and collaborate securely — all within a single, unified platform.
With advanced lifecycle management, real-time validation, and AI-assisted insights, DnXT Reviewer empowers regulatory professionals to focus on what matters most — submission quality, speed, and compliance.
🔹 Key Highlights:
Intelligent eCTD viewing and lifecycle tracking
Rapid navigation with contextual cross-referencing
Secure annotation, collaboration, and audit trails
Built-in compliance with regional eCTD standards (US, EU, CA, etc.)
Cloud-ready architecture for scalability and remote access
DnXT Reviewer — your smarter path to regulatory review excellence.

04
Integrated AI
DnXT AI — Powering the Future of Intelligent Regulatory Operations
Meet DnXT AI, the intelligent core of next-generation regulatory transformation. Purpose-built for life sciences, DnXT AI seamlessly integrates advanced artificial intelligence into every stage of the regulatory lifecycle — from authoring and submission to review and compliance.
With DnXT AI, regulatory teams move beyond manual effort to true digital intelligence. The platform automates document classification, validates submissions in real time, predicts compliance risks, and even assists reviewers with contextual insights — all within one unified ecosystem.
🔹 Smarter Submissions: AI-driven document tagging, metadata validation, and error detection
🔹 Accelerated Reviews: Automated sequence comparisons and contextual content summarization
🔹 Predictive Compliance: Early alerts on regulatory changes and risk impacts
🔹 Seamless Integration: Works natively across the DnXT platform for end-to-end efficiency
DnXT AI isn’t just automation — it’s intelligence that understands regulation.
Transform your regulatory operations with a platform that learns, adapts, and empowers.
DnXT AI — Smarter. Faster. Compliant.

Services
Building Trusted Partnership with Bold Execution
DNXT Services specializes in simplifying complex process to make any task more intuitive and easy to execute. We work closely with our clients to handle various aspects of the process, technology and high level objectives allowing clients to concentrate on their scientific innovations. The services provided by DNXT include Strategy & Advisory, End to End System Implementation, Program/Project Management, Business Process, Regulatory Publishing Services and Custom Software Development whether you need an integration or decide you need something better than what is available in the market.
Our work isn’t just about creating - it’s about making a difference.
Let's make anImpact together
CONTACTS
Let's work in your vision
We would be delighted to assist you! Whether you are seeking a demo, an evaluation, or simply want to learn more about how our regulatory publishing and strategy services can benefit your organization, please contact us for a complimentary consultation. We are committed to helping you navigate the regulatory landscape with confidence and efficiency.
OUR CLIENTS FEEDBACK
WE ARE DnXT
ABOUT US
We are passionate about innovation. We want to build efficient solutions together. Our motto is to grow together. We want to be your trusted partner in life science.