Automated Assembly
Drag-and-drop dossier building with intelligent document ordering and lifecycle management
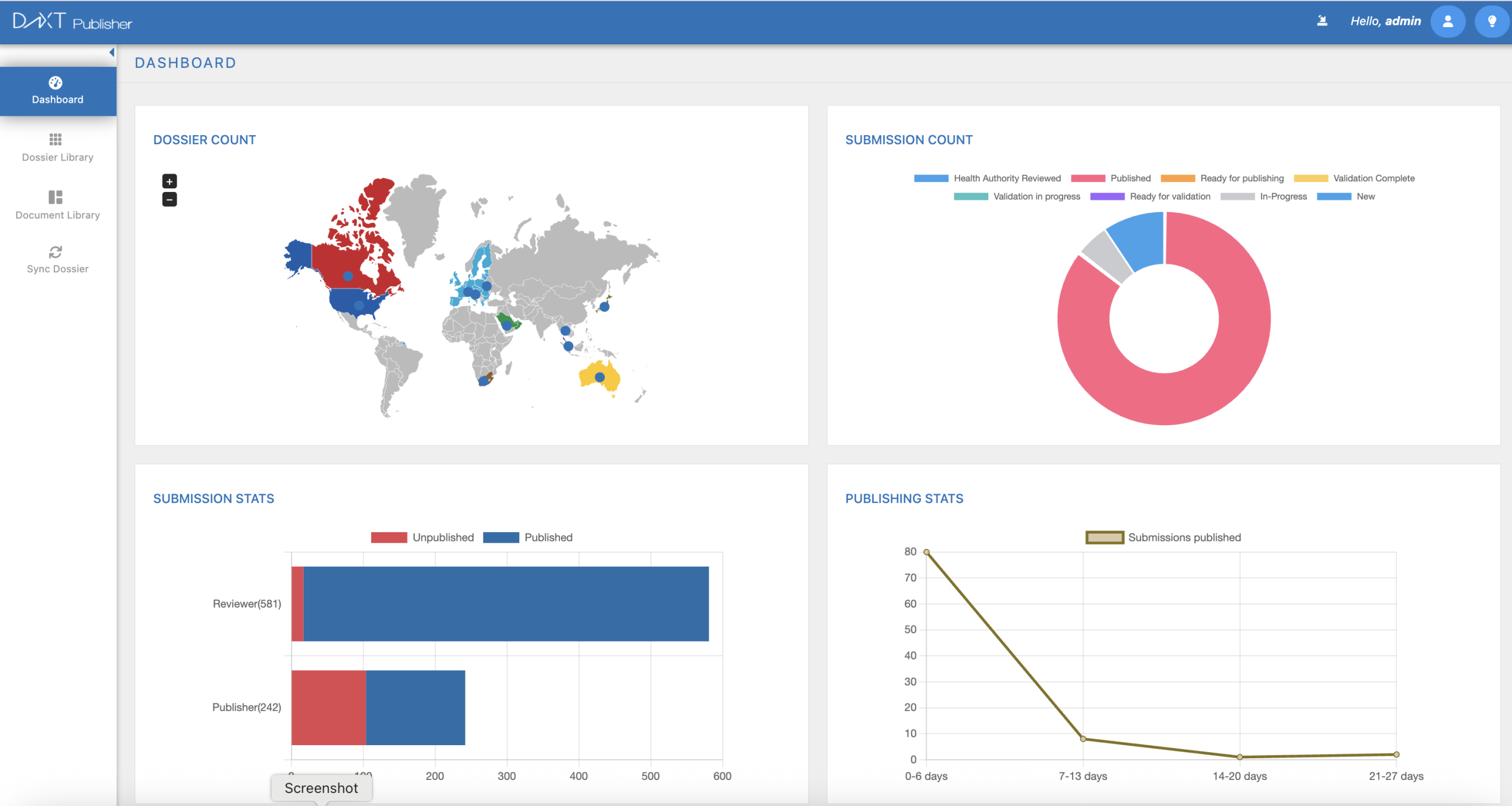
Transform complex dossier assembly into a streamlined, automated process. DnXT Publisher delivers compliant, submission-ready eCTD packages with real-time validation, automated metadata management, and direct DMS integration — so your team can focus on strategy, not formatting.
Drag-and-drop dossier building with intelligent document ordering and lifecycle management
Seamless connection to Veeva Vault, SharePoint, and other document management systems
Catch errors before submission with built-in eCTD validation against all regional specifications
Publish to FDA, EMA, Health Canada, TGA, and more from a single platform
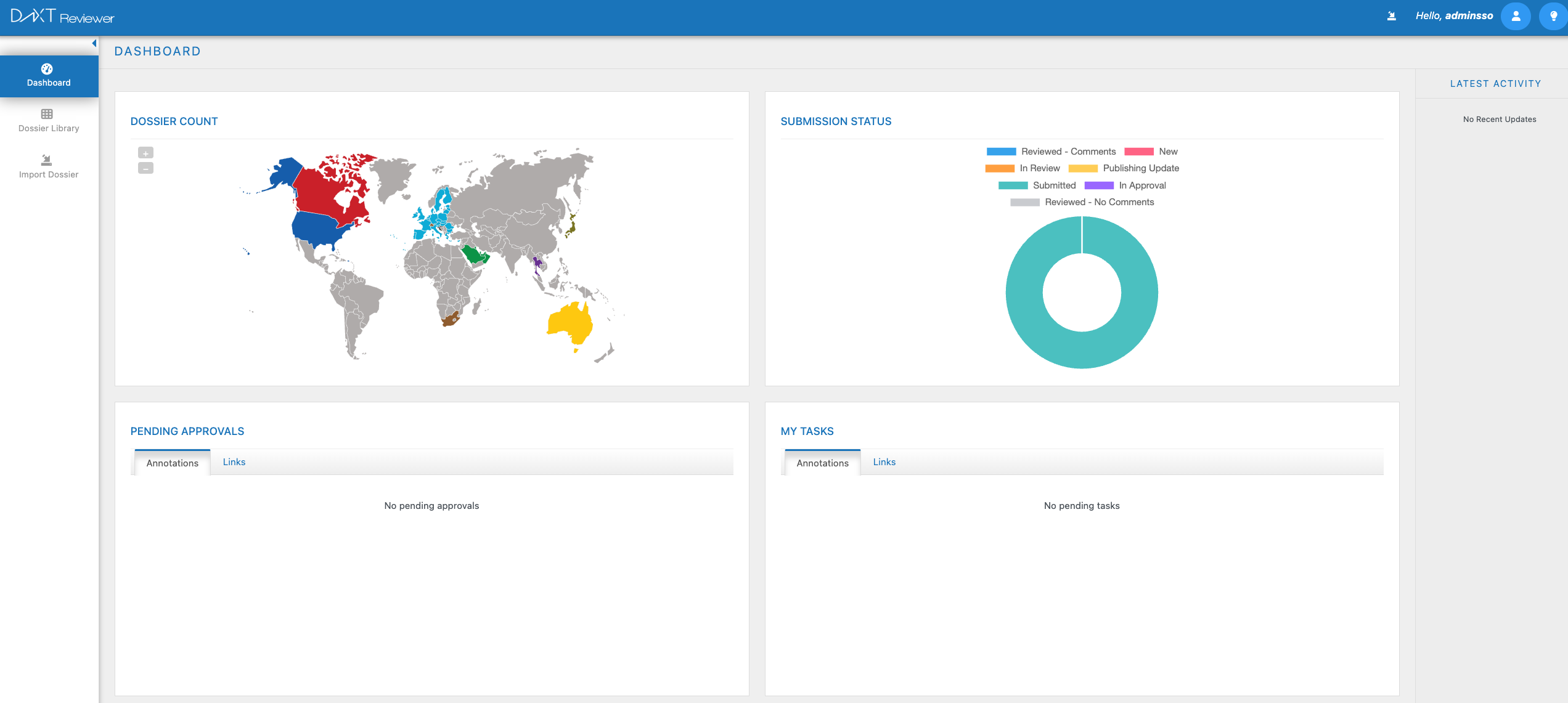
Navigate complex eCTD submissions with an intuitive, cloud-native review experience. Compare sequences in seconds, collaborate securely across teams, and maintain complete audit trails — all within a single, unified platform built for regulatory professionals.
Intelligent eCTD viewing with full lifecycle history and cross-reference navigation
Annotation, comments, and review workflows with role-based access control
Rapid side-by-side comparison of submission sequences with change highlighting
Direct comparison of labeling content across previous lifecycles for faster QC

A comprehensive Regulatory Information Management suite that treats regulatory operations as a strategic asset, not administrative overhead. Plan submissions, track registrations, manage correspondence, and orchestrate your entire regulatory lifecycle from a single command center.
Visual timeline management with milestone tracking, dependencies, and agency deadline monitoring
Centralized tracking of agency communications with automated response workflows
Global registration status dashboard with country-level visibility and renewal alerts
Seamlessly connect with CROs, affiliates, and external partners for controlled outsourcing

Purpose-built AI that seamlessly integrates into every stage of the regulatory lifecycle. From automated document classification and real-time validation to predictive compliance alerts and contextual review assistance — DnXT AI transforms manual effort into digital intelligence.
AI-driven tagging, metadata extraction, and automatic CTD section mapping
Early alerts on regulatory changes, risk impacts, and potential submission gaps
Contextual summaries of submission documents and sequence changes for faster review
Automated parsing and classification of agency correspondence with action item extraction
Platform Capabilities
Each capability is built for regulatory teams who need speed, compliance, and control — from first draft to agency submission.